Combination of tensile stress and specific corrosive environment may rupture stainless steel plates
- Wilson Pipeline
- Jan 5, 2022
- 3 min read
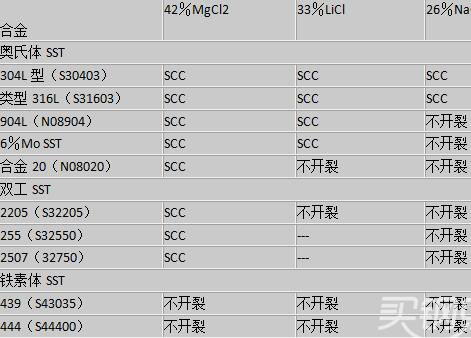
The combination of tensile stress and a specific corrosive environment can cause the stainless steel plate to break. This type of attack is called Stress Corrosion Cracking (SCC). The most common environmental exposure condition that causes SCC in stainless steel is the presence of chloride. Although the degree of stainless steel immunity to chloride SCC is large, the relative resistance of stainless steel plates varies greatly. Effect of alloy composition: The relative resistance of chloride SCC depends on the stainless steel series. The austenitic stainless steel series is the most susceptible. The tolerance of SCC to austenitic stainless steels is related to the nickel content. The austenitic stainless steels that are most susceptible to SCC have a nickel content in the range of 8-10%. Therefore, standard brands such as 304, 304L and 316, 316L are very sensitive to this type of attack. Austenitic grades with higher nickel and molybdenum contents, such as alloy 20, 904L and 6% molybdenum superaustenitic grades have significantly better chloride SCC resistance. The ferritic stainless steel series includes grades 430 and 444 and is very resistant to chloride SCC. Duplex stainless steels with austenitic, ferrite microstructures have electrical resistance between the austenitic and ferritic grades. Corrosion test The relative resistance of the stainless steel to the chloride SCC is usually quantified by using a standard boiling salt solution. The following table summarizes the test results in 26% NaCl (sodium chloride), 33% LiCl (lithium chloride) and 42% MgCl 2 (magnesium chloride) in boiling salt solution. Boiling LiCl and MgCl 2 test solutions are very aggressive with respect to practical use, and only austenitic alloys with compositions close to that of nickel-based alloys will routinely resist cracks in these test solutions. Table 1: Relative chloride chloride SCC resistance measured in a standard boiling salt solution using a fully immersed U-bend specimen. (taken from producer data)
Cracked appearance:
The typical crack morphology of chloride stress corrosion cracking consists of branching nucleation cracks. Figure 1 shows the cracks that occur on 6Mo super austenitic stainless steel (N08367) exposed to 0.2% chloride at 500°F (260°C).
Figure 1: Typical appearance of chloride stress corrosion cracking

Envirnmental factor:
Environmental factors that increase the susceptibility of stainless steel to cracking include higher temperatures, increased chloride content, lower pH, and higher tensile stress levels. Temperature is an important variable. When the stainless steel plate is completely submerged, chloride stress corrosion cracking of the stainless steel plate is rarely seen at temperatures below 60°C.
There is a synergistic relationship between dissolved oxygen and chloride levels. If the oxygen content is reduced to the 0.01-0.1 ppm range, aqueous solutions containing low to moderate chloride levels cannot crack austenitic stainless steels, such as 304L and 316L stainless steels. At room temperature to moderate temperatures, the normal solubility of oxygen in water is 4.5-8 ppm at atmospheric pressure.
In actual use environments, evaporation can cause local accumulation of corrosive substances such as chlorides and H+ ions, leading to severe corrosive conditions. Under severe evaporation conditions, stainless steel plates will crack at temperatures well below the threshold measured under full immersion conditions. Therefore, care must be taken when specifying materials for applications involving the evaporation of chlorine-containing solutions on the surface of hot stainless steel plates.
Figure 1 shows the crack threshold for 304L and 316L stainless steel as a function of temperature and chloride content. The amount of chloride needed to produce cracks is relatively low. Failure has occurred in environments with as little as 10 ppm chloride. This is especially true of environments with a concentrated (evaporation) mechanism (eg, a wet/dry interface) or a solution film that is in immediate contact with a heat-resistant surface. In these cases, a few ppm of chloride in the bulk solution can be concentrated to several hundred ppm in the evaporation zone.
Figure 1: Cracking thresholds for 304 and 316 alloys exposed to near-neutral chlorine-containing water
The crack threshold of 6Mo super austenitic stainless steel (UNS N08367) immersed in an oxygen-containing neutral chloride solution is shown in FIG. 2 . The temperature threshold is much higher than the 100°C range, indicating that exposure to the atmosphere is unlikely to cause cracking when boiled in a neutral chloride solution.
Source: China Stainless Steel Plates Manufacturer – wilsonpipeline Pipe Industry Co., Limited (www.wilsonpipeline.com)

Comments